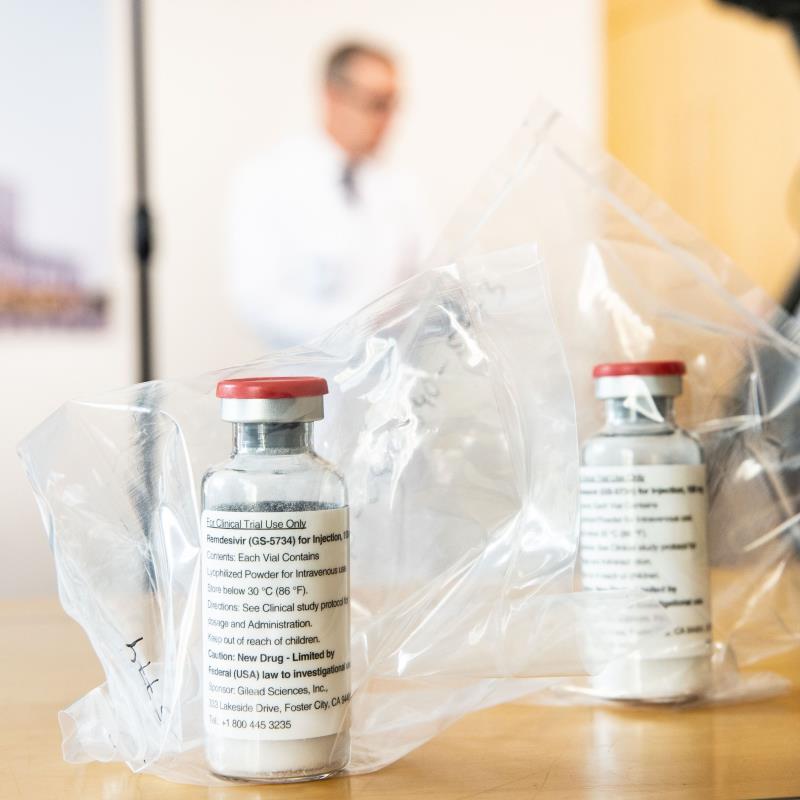
The FDA announced Friday that its emergency use authorization for experimental drug remdesivir to treat COVID-19 had expanded to include all hospitalized patients, one week after new research indicated the drug may be effective against moderate illness.




More Stories
Brazil rolls out COVID-19 vaccination plan
First Pfizer COVID-19 vaccine shipments to be delivered to US distribution sites starting Monday
CDC advisory panel recommends Covid-19 vaccine for people 16 and over