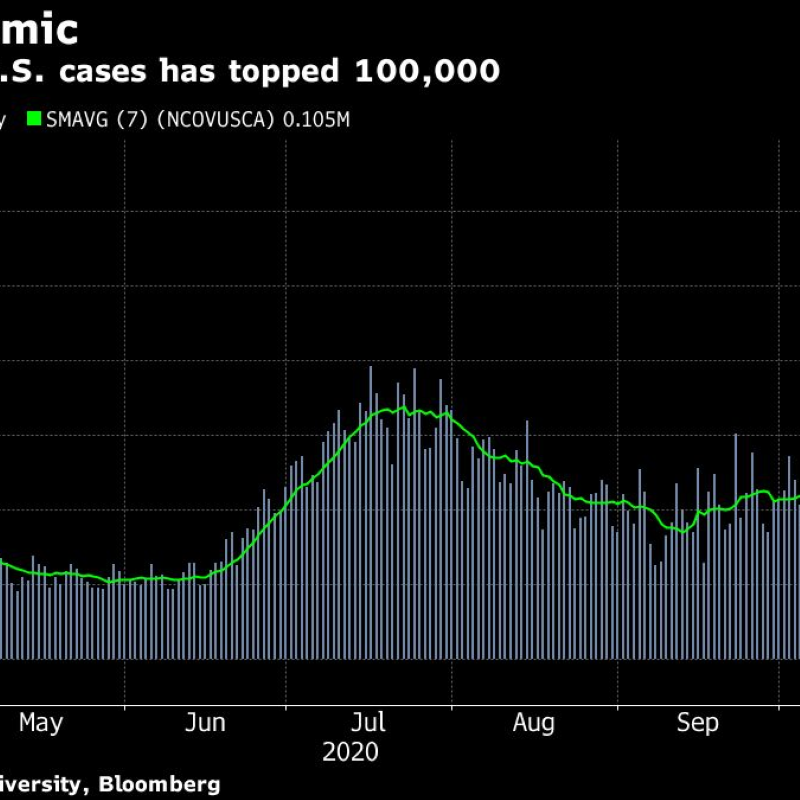
Pfizer and BioNTech reported that their vaccine has prevented more than 90% of infections in an interim report on its final-stage trial, and Eli Lilly’s new antibody therapy received emergency use approval from U.S. regulators, widening access to a treatment for mild to moderate cases in adults and children.




More Stories
Record Korean Cases; Trader’s Guide to Rollout: Virus Update
California Hits Record; Trader’s Guide to Rollout: Virus Update
AstraZeneca to Buy Alexion for $39 Billion in Rare-Disease Push