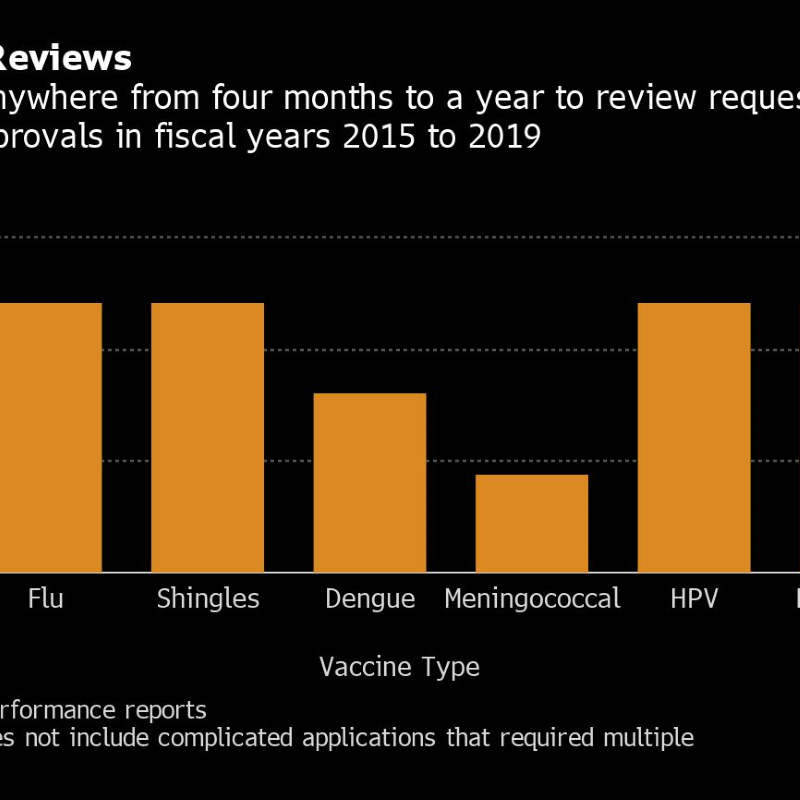
Pfizer Inc. and BioNTech SE are on the brink of requesting emergency authorization of their Covid vaccine, and it could take at least three weeks for a U.S. Food and Drug Administration decision as trial data is probed by agency staff and outside advisers.




More Stories
Record Korean Cases; Trader’s Guide to Rollout: Virus Update
California Hits Record; Trader’s Guide to Rollout: Virus Update
AstraZeneca to Buy Alexion for $39 Billion in Rare-Disease Push